What Are Food Contact Materials?
Food Contact Materials (FCMs) are materials that are already or are intended to be in contact with food and beverages. FCMs also includes materials that can reasonably be expected to be in contact with food under normal or foreseeable use. Even though, these materials are designed to improve food hygienic and ease its handling and consumption, this is an important aspect for food and beverage manufacturers as the constituents of these materials could be transferred to the food. In other words, these materials’ molecules could migrate to the food during direct or indirect contact, and alter quality in food manufacturing. It concerns every step of the supply chain from production to serving, as a product can be in contact with various packaging, machinery and containers. FCMs include various types of materials, such as plastic, aluminum, coatings, and metal. As a result, many countries have implemented regulations to ensure food safety.
Regulations and Food Contact Materials
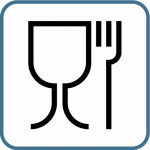
This wine glass and fork icon is the international labeling symbol, indicating that a product is made of materials designed for contact with food. It means that it can be safely used for food. However, specific regulations may vary from one country to another:
USA
In the US, the Food and Drug Administration (FDA) is responsible to set rules and guidelines regarding the material composition, properties and uses. The regulatory status of a FCM depends of the regulatory status of each individual constituent of this material. These constituents are regulated as indirect food additive, – substances that might be transferred to the food by its packaging or processing equipment -, under the Food Drug and Cosmetics Act (FD&C Act) and Title 21 Code of Federal Regulations. The FDA first defines if each food contact substance is safe, and, then, approves a food contact materials only if all its constituents are given FDA clearance.
There are three main steps to be FDA compliant. The first one concerns the established FCMs, which can follow existing specific FDA regulations. The second step is for new food contact substances that require authorization from the FDA; businesses need to submit a Food Contact Notification (FCN) and/or Threshold of Regulation (ToR). Finally, if a company can argue that there is no need to notify the substance, it can use the No Migration Exemption.
Canada
In Canada, Division 23 of the Food and Drug Act and Regulations governs the food contact materials. Section B.23.001 “prohibits the sale of foods in packages that may impart any substance to the contents which may be harmful to the consumer of the food”. However, manufacturers and distributors do not have direct compliance responsibility. If they are confident that their FCMs are safe, they are not obligated to submit a food contact notification or any test results. Nonetheless, they can, on a voluntary-basis, request pre-market assessment and a letter of no objection for their products, from the Health Products and Food Branch (HPFFB).
It is interesting to note that, in Canada, food contact materials are those in contact with food only during manufacturing and processing, but it does not include items such as kitchen tools, utensils, etc.
Europe
In Europe, the Commission Regulation (EC) 1935/2004 is the main reference for all food contact materials. Article 3 states that substances present in FCMs shall not migrate into food in concentrations that could endanger human health or imply an unacceptable change in the food composition or a deterioration in its organoleptic properties. This regulation also requires FCMs manufacturing to comply with the Good Manufacturing Practices (GMP) laid down in Commission Regulation (EC) 2023/2006. In addition, the materials should undertake appropriate tests and might be subject to a Declaration of Compliance (DoC).
Regarding finished plastic materials and articles, they must follow the Regulation (EU) 10/2011. Regulations can vary from one European country to another in the absence of a specific EU measures. They can have their own national provisions that comply with the rules of the treaty.
Ensure Compliance of Your Food Contact Materials
Regulatory Experts and IT solutions, such as a Product Lifecycle Management software (PLM), can help you navigate through all these country-specific regulations, and support product compliance. Regulatory affairs can be complex and having the right tools and skills are crucial to be regulatory-compliant. Discover how Lascom – PLM solution Food & Beverage – is the right software to help you ensure product compliance, from marketing brief to a product’s end of life.