FSMA has a core framework that rests on four key pillars.
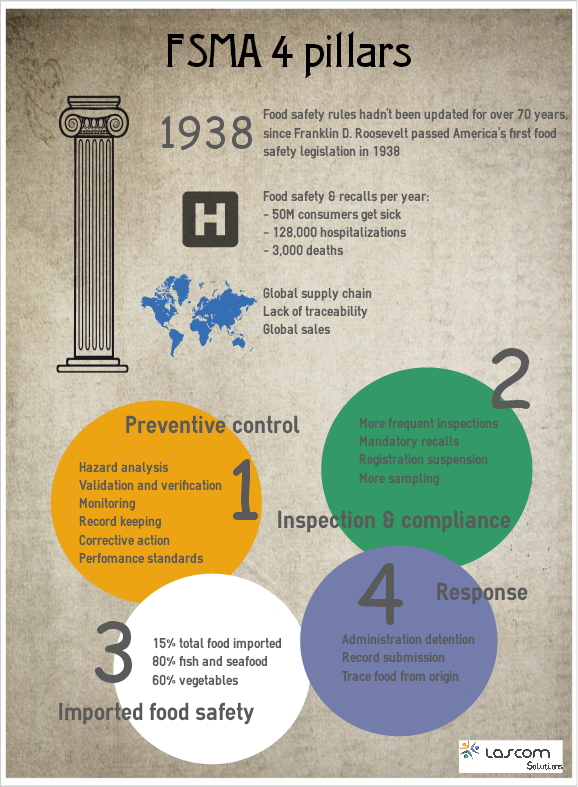
Preventive Controls
- Hazard Analysis: You'll need to have plans in place to identify food safety hazards (any property or condition that may make food unsafe) in your facilities and determine preventive measures that can be applied to control these hazards.
- Validation and Verification: It's great to have a Hazard Analysis plan, but it is no good if it is not working. You'll need strong validation and verification plans and procedures that effectively ensure your system is working as intended.
- Monitoring: This involves essentially defining the monitoring activities that will be undertaken to ensure defined critical control point processes are effective and provably under control.
- Recordkeeping: This is a no-brainer. When FSMA is fully revealed, it is going to be absolutely integral for food companies to maintain comprehensive records. While paper records can be retained to attempt to meet recordkeeping requirements, this approach will be incredibly time-consuming, costly, reactive and fundamentally insufficient to meet FSMA requirements. Centralized, web-based document control solutions will be the most effective way to proactively manage compliance.
- Corrective Actions and Performance Standards: Food companies must increase the volume, detail and availability of records related to monitoring, corrective actions and verification and these records must be provided to the FDA upon request.
Inspection and Compliance
- There is an inspection frequency mandate that is based on risk:
- High-risk facilities will be inspected once in the first five years of the signing of the Act.
- These facilities will be inspected once every three years thereafter.
- Others (i.e. not identified as high-risk) will be inspected once in the first seven years of the signing of the Act, and once every 5 years thereafter.
- Mandatory recalls
- Registration suspension: The FDA also has the ability to suspend a registration, essentially disallowing producers from distributing a product from a facility where registration has been suspended. This applies to both importing and exporting products into/out of the United States.
- More sampling: In addition to increased inspections, there will also be increased, more intensive sampling and testing of products. The whole idea behind many of these FSMA inspection and compliance aspects is that the Administration needs to be able to trace back exactly where food came from in the event of an outbreak and understand the analysis that was performed on the food product as it went through food supply chain.
Imported Food Safety
The more rigorous rules on food imports are rooted in expectation that food consumed is safe to eat regardless of where it comes from. In today's global food supply chain, more and more food is imported into America: 15% overall, 60% when it comes to food and vegetables, and 80% of fish and seafood.
Response
As the most regulation-light facet of the Act, many provisions laid out in the response component of FSMA have already been implemented and enforced. The key elements of FSMA's response powers, includes:
- Mandatory recall powers.
- Expanded administrative detention.
- Record submission.
In addition the FDA has also launched a product tracing project that will enhance its ability to track and trace both domestic and imported foods, and is also implementing additional recordkeeping rules for high-risk foods